(a)
Considering the given information:
Identify the given values first.
5.00 volumes of and 1.00 volumes of means that the reactant has a 5 : 1 -mole ratio. Solve for the mole fraction of each reactant.
At equilibrium, the total pressure of the reaction is. Multiply the mole ratios of each reactant to find the partial pressures of each.
Write the reaction table, next:
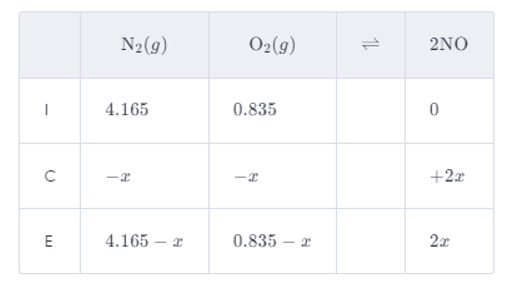
In terms of partial pressures, write the expression for the reaction's equilibrium constant.
Using the reaction table and the given , solve for x. Because the is small, we can remove the x from the denominator.
Calculate the partial NO reaction.


