Amino acids [general formula can be considered polypro tic acids. In many cases, the R group contains additional amine and carboxyl groups.
(a) Can an amino acid dissolved in pure water have a protonated localid="1663345833873" group and an unprotonated localid="1663345865389" group
localid="1663345870225"
Use glycine, localid="1663345879880" , to explain why.
(b) Calculate localid="1663345908281"
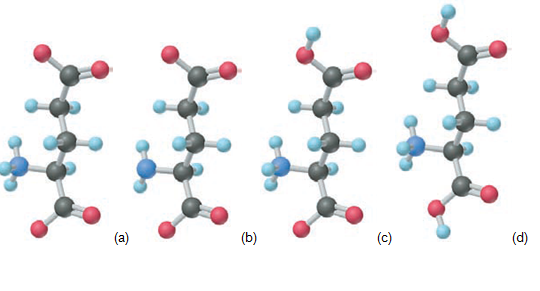
(c) The R group of lysine is localid="1663345894686" Draw the structure of lysine at .localid="1663345916050" physiological localid="1663345902202"
(d) Thelocalid="1663345947279" group of glutamic acid localid="1663345920800" of the forms of glutamic acid that are shown below, which predominates at,localid="1663345941655" localid="1663345993071" (2) localid="1663345925494"
and (3) localid="1663345936358"