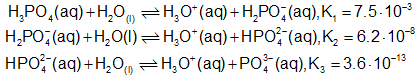Let’s consider, \(\left( {{{\rm{H}}^ + }} \right)\) in the urine to be able to predict dissociation of other ions.
\(\begin{array}{*{20}{c}}{{\rm{pH}} = - \log \left( {{{\rm{H}}^ + }} \right)}\\{6.3 = - \log \left( {{{\rm{H}}^ + }} \right)}\\{\log \left( {{{\rm{H}}^ + }} \right) = - 6.3}\end{array}\)
\(\begin{array}{*{20}{c}}{\left( {{{\rm{H}}^ + }} \right) = {\rm{antilog\;}}( - 6.3)}\\{\left( {{{\rm{H}}^ + }} \right) = 5.01 \cdot {{10}^{ - 7}}}\end{array}\)
Dissociation of Phosphate,
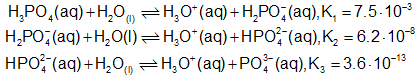
\({{\rm{K}}_3} = \frac{{\left( {{{\rm{H}}_3}{{\rm{O}}^ + }} \right)\left( {{\rm{PO}}_4^{3 - }} \right)}}{{\left( {{\rm{HPO}}_4^{2 - }} \right)}}\)
\(\begin{array}{*{20}{c}}{3.6 \cdot {{10}^{ - 13}} = \frac{{5.01 \cdot {{10}^{ - 7}}\left( {{\rm{PO}}_4^{3 - }} \right)}}{{\left( {{\rm{HPO}}_4^{2 - }} \right)}}}\\{\left( {{\rm{HPO}}_4^{2 - }} \right) = \frac{{5.01 \cdot {{10}^{ - 7}}}}{{3.6 \cdot {{10}^{ - 13}}}}\left( {{\rm{PO}}_4^{3 - }} \right)}\end{array}\)
\(\left( {{\rm{HPO}}_4^{2 - }} \right) = 1.39 \cdot {10^6}\left( {{\rm{PO}}_4^{3 - }} \right)\)
So, the concentration is expressed as \(\left( {{\rm{PO}}_4^{3 - }} \right){\rm{\;is\;}}1.39 \cdot {10^6}\left( {{\rm{PO}}_4^{3 - }} \right)\).