For the past 10 years, the unsaturated hydrocarbon 1,3-butadiene \(\left( {{\bf{C}}{{\bf{H}}_{\bf{2}}}{\bf{ = CH - CH = C}}{{\bf{H}}_{\bf{2}}}} \right)\) has ranked 38th among the top 50 industrial chemicals. It is used primarily for the manufacture of synthetic rubber. An isomer exists also as cyclobutene:
![]()
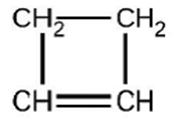
The isomerization of cyclobutene to butadiene is first-order, and the rate constant has been measured as \({\bf{2}}{\bf{.0 \times 1}}{{\bf{0}}^{{\bf{ - 4}}}}{{\bf{s}}^{{\bf{ - 1}}}}\) at 150 \({\bf{^\circ C}}\) in a 0.53-L flask. Determine the partial pressure of cyclobutene and its concentration after 30.0 minutes if an isomerization reaction is carried out at 150 \({\bf{^\circ C}}\) with an initial pressure of 55 torr.



