Question. Pyrrole undergoes electrophilic aromatic substitution more readily than benzene, and mild reagents and conditions are sufficient. These reactions normally occur at the 2-position rather than the 3-position, as shown in the following example.
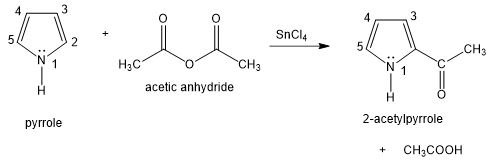
- Propose a mechanism for the acetylation of pyrrole just shown. You may begin with pyrrole and the acylium ion,
 . Be careful to draw all the resonance structures of this intermediate.
. Be careful to draw all the resonance structures of this intermediate. - Explain why pyrrole reacts more readily than benzene, and also why substitution occurs primarily at the 2-position rather than 3-position.










 . Be careful to draw all the resonance structures of this intermediate.
. Be careful to draw all the resonance structures of this intermediate.





