Chapter 26: Q 39. (page 1072)
The reaction of cyclohexene with iodo-benzene under Heck conditions forms E, a coupling product with the new phenyl group on the allylic carbon, but none of the “expected” coupling product F with the phenyl group bonded directly to the carbon–carbon double bond.
a. Draw a stepwise mechanism that illustrates how E is formed.
b. Step [2] in Mechanism 26.2 proceeds with syn addition of Pd and R' to the double bond.What does the formation of E suggest about the stereochemistry of the elimination reaction depicted in Step [3] of Mechanism 26.2?

Short Answer
Answer
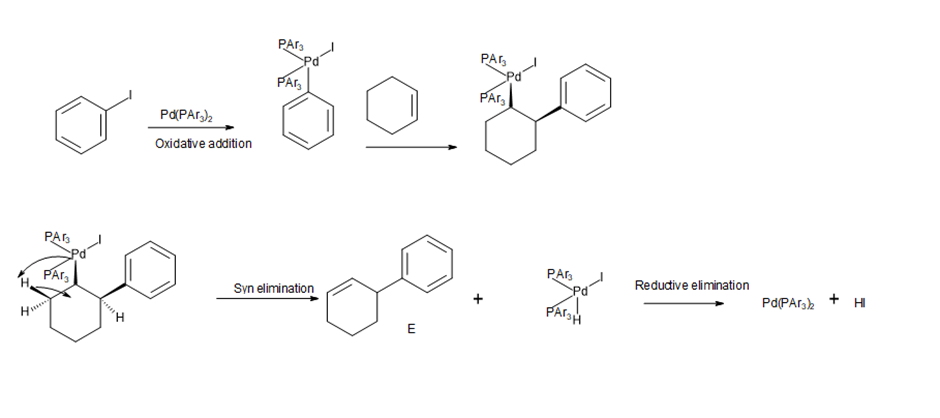
 Heck reaction
Heck reaction Step 1
Step 1 Step 2
Step 2 Step 3
Step 3 The product that is not formed
The product that is not formed Step 4
Step 4 mechanism of Heck reaction
mechanism of Heck reaction







