(a)The reaction of butan-1-ol with TsCl and pyridine leads to butyl tosylate. The butyl tosylate further reacts with NaBr to generate 1-bromobutane.The reaction can be given as:
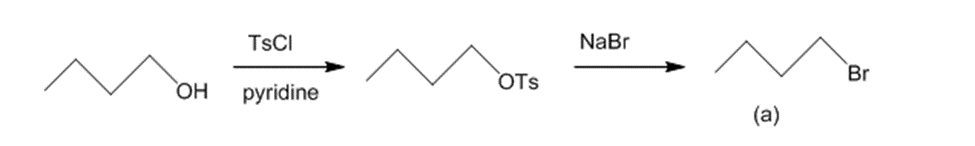
Conversion of butan-1-ol to 1-bromobutane
(b) The reaction of butan-1-ol with TsCl and pyridine leads to butyl tosylate. The butyl tosylate further reacts with excess ammonia to generate butan-1-amine.The reaction can be given as:
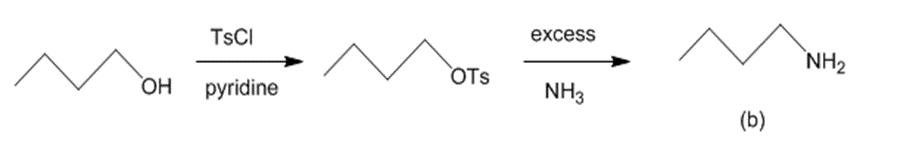
Conversion of butan-1-ol to butan-1-amine
(c) The reaction of butan-1-ol with TsCl and pyridine leads to butyl tosylate. The butyl tosylate further reacts with NaOCH2CH3 generate butyl ethyl ether.The reaction can be given as:
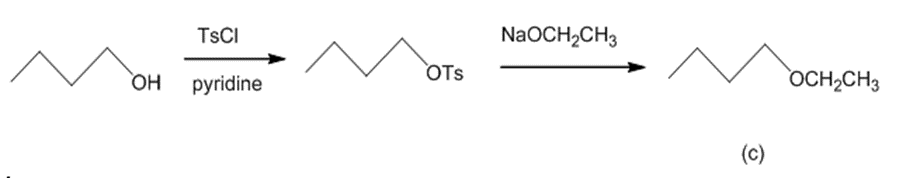
Conversion of butan-1-ol to butyl ethyl ether
(d) The reaction of butan-1-ol with TsCl and pyridine leads to butyl tosylate. The butyl tosylate further reacts with KCN to generate pentannitrile. The reaction can be given as:
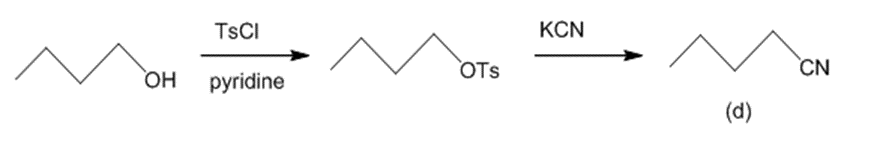
Conversion of butan-1-ol to pentannitrile















