Chapter 11: Q26P (page 570)
Propose a mechanism for each reaction.(a)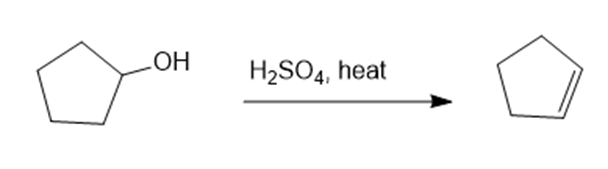
(b)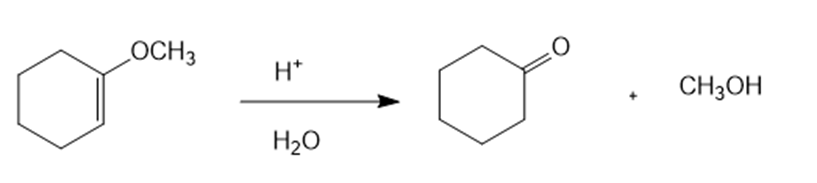
(c)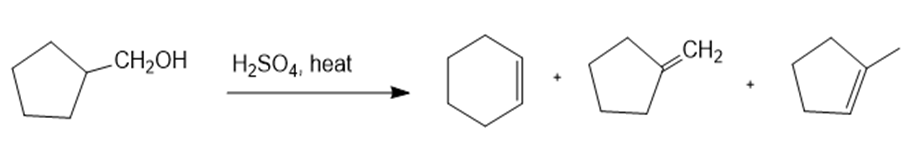
(d) 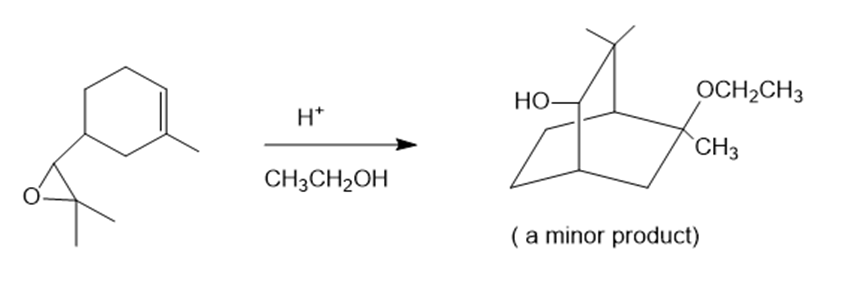
(a)
(b)
(c)
(d) 
Short Answer
(a) 
(b)
(c) 
(d) 
Chapter 11: Q26P (page 570)
(a)
(b)
(c)
(d) 
(a) 
(b)
(c) 
(d) 
All the tools & learning materials you need for study success - in one app.
Get started for free
Alcohols combine with ketones and aldehydes to form interesting derivatives, which we will discuss in Chapter 18. The following reactions show the hydrolysis of two such derivatives. Propose mechanisms for these reactions.
(a)

(b)

Under normal circumstances, tertiary alcohols are not oxidized. However, when the tertiary alcohol is allylic, it can undergo a migration of the double bond (called an allylic shift) and subsequent oxidation of the alcohol. A particularly effective reagent for this reaction is Bobbitt’s reagent, similar to TEMPO used in many oxidations. (M. Shibuya et al., J. Org. Chem., 2008, 73, 4750.)

Show the expected product when each of these 3° allylic alcohols is oxidized by Bobbitt’s reagent.
(a)

(b)

(c)

(d)

Predict the products you expect when the following starting material undergoes oxidation with an excess of each of the reagents shown below.

(a)chromic acid
(b)PCC(pyridinium chlorochromate)
(c)sodium hypochlorite/ acetic acid
(d)DMSO and oxalyl chloride
(e)DMP(periodinane)reagent
Two unknowns, X and Y, both having the molecular formula C4H8O , give the following results with four chemical tests. Propose structures for X and Y consistent with this information.
Bromine | Na metal | Chromic acid | Lucas reagent | |
Compound X | decolorizes | Bubbles | orange to green | no reaction |
Compound Y | no reaction | no reaction | no reaction | no reaction |
A student wanted to use the Williamson ether synthesis to make (R)-2-ethoxybutane. He remembered that the Williamson synthesis involves an SN2 displacement, which takes place with inversion of configuration. He ordered a bottle of (S)-butan-2-ol for his chiral starting material. He also remembered that the SN2goes best on primary halides and tosylates, so he made ethyl tosylate and sodium (S)-but-2-oxide. After warming these reagents together, he obtained an excellent yield of 2-ethoxybutane.
(a) What enantiomer of 2-ethoxybutane did he obtain? Explain how this enantiomer results from the SN2 reaction of ethyl tosylate with sodium (S)-but-2-oxide.
(b) What would have been the best synthesis of (R)-2-ethoxybutane?
(c) How can this student convert the rest of his bottle of (S)-butan-2-ol to (R)-2-ethoxybutane?
What do you think about this solution?
We value your feedback to improve our textbook solutions.