(a) When propanol is treated with 1 equivalent of NaOCl, it forms propanal. Aldehydes, when reacted with Grignard reagent, form secondary alcohol. Therefore, propanal reacts with ethyl magnesium bromide followed by hydrolysis from pentan-3-ol. Pentan-3-ol is treated with NaOCl and HOAc to form pentan-3-one.
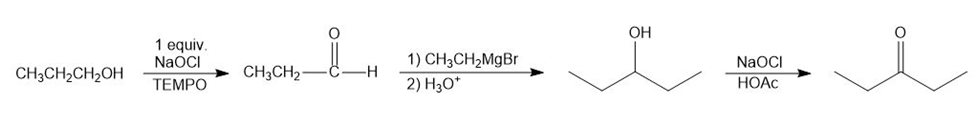
Formation of pentan-3-one
(b) When propanoyl chloride is treated with ethyl magnesium bromide followed by hydrolysis form 3-ethyl-pentan-3-ol. 3-ethyl-pentan-3-ol treated with sulphuric acid undergoes dehydration to form 3-ethyl-pentan-2-ene, which on further reaction with BH3. THF and H2O2, HO- form 3-ethyl-pentan-2-ol. 3-ethyl-pentan-2-ol treated with NaOCl and HOAc forms 3-ethylpentan-2-one.
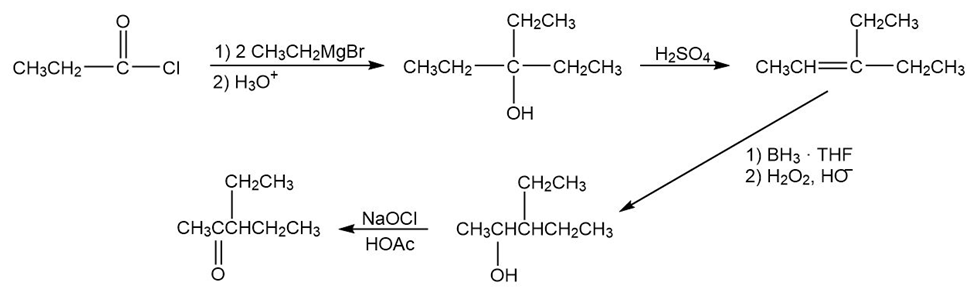
Formation of 3-ethylpentan-2-one












