(a)The primary alcohol is oxidized to carboxylic acid when the starting material is treated with chromic acid (H2CrO4). The reaction can be given as:
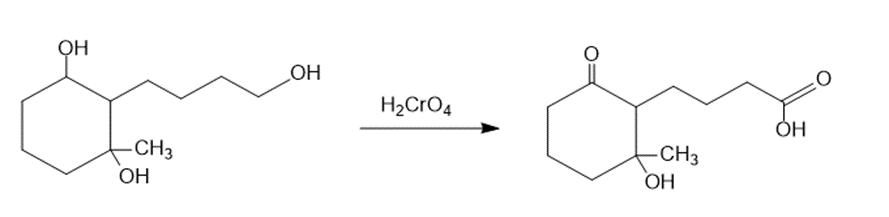
The treatment of the starting material with chromic acid
(b)The primary alcohol is oxidized into an aldehyde when the starting material is treated with PCC. The reaction can be given as:
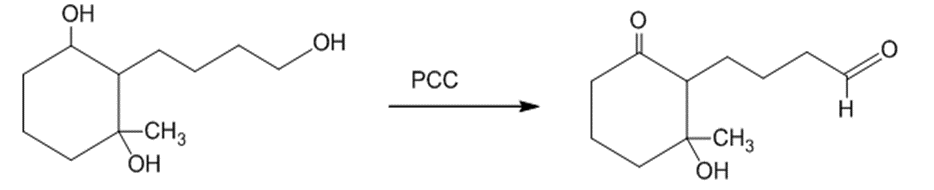
The treatment of the starting material with PCC
(c)The treatment of the starting material with NaOCl oxidizes the primary alcohol into a carboxylic acid. The reaction can be given as:
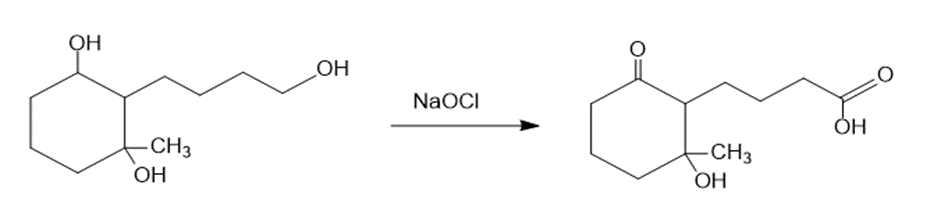
The treatment of the starting material with NaOCl
(d)The treatment of the starting material with DMSO or oxalyl chloride oxidizes the primary alcohol into an aldehyde. The reaction can be given as:
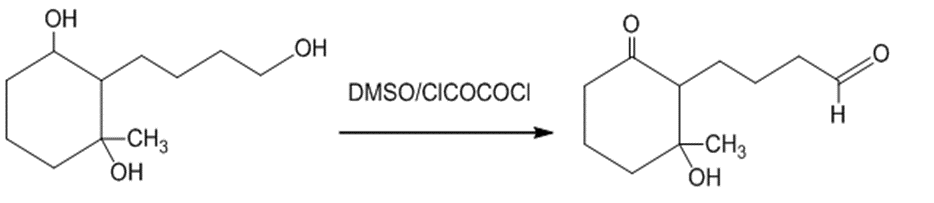
The treatment of the starting material with DMSO or oxalyl chloride
(e)The treatment of the starting material with DMP oxidizes the primary alcohol into an aldehyde. The reaction can be given as:
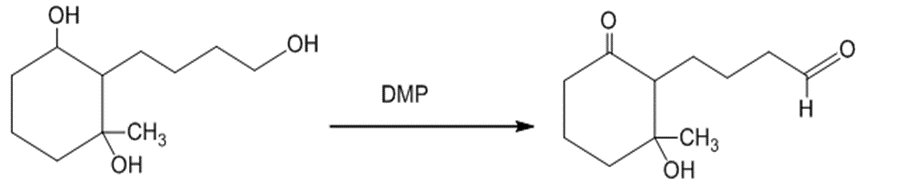
The treatment of the starting material with DMP














