(a)The heptan-1-ol is formed by the reaction of heptanal either NaBH4 in the presence of CH3OH or by the reaction LiAI4 with H3O+
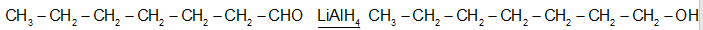
Heptan-1-al heptan-1-ol
b. The heptan-2-ol is formed by the reaction of 2 heptanone either NaBH4 in the presence of CH3OH or by the reaction LiAI4 with H3O+
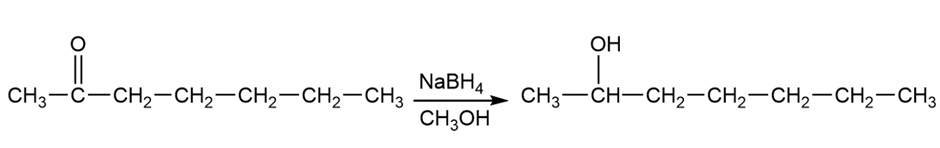
2-heptanone heptan-2-ol
c.The 2-methyl hexan-3 ol is formed by the reaction of 2-methyl-3-hexanone either NaBH4 in the presence of CH3OH or by the reaction LiAIH4 with H3O+
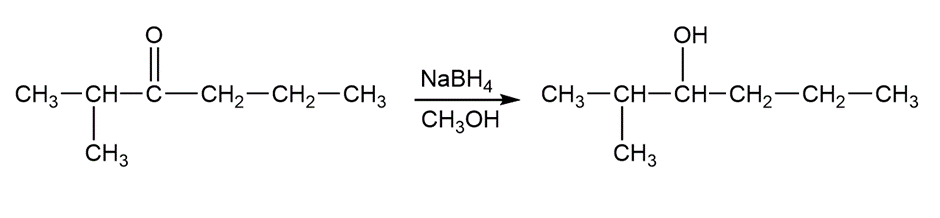
2-methyl-3-hexanone 2-methyl hexan-3-ol
d. In this reaction, the desired product will not be formed by the reaction of the reactant LiAIH4 as it also reduces the ester group in addition to the ketone group whereas the NaBH4 will reduce only the ketone group. So, this reaction is possible only with NaBH4
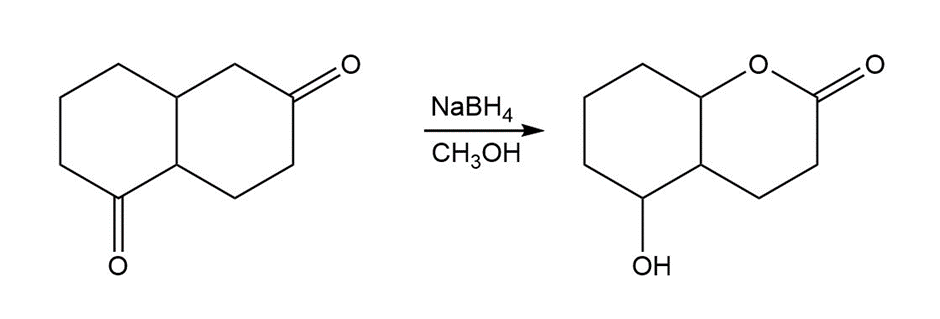





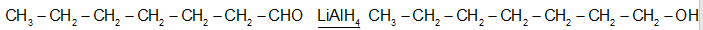





 CH
CH









