Chapter 16: Q81P (page 796)
- Propose a mechanism for the following reaction:
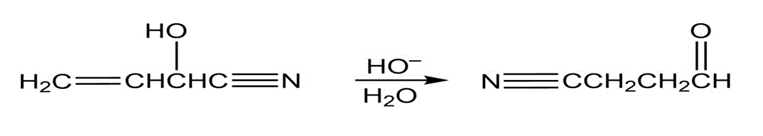
- What is the product of the following reaction?
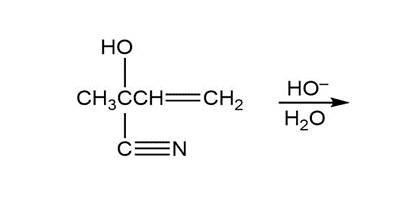


Chapter 16: Q81P (page 796)




All the tools & learning materials you need for study success - in one app.
Get started for free
When a cyclic ketone reacts with diazomethane, the next larger cyclic ketone is formed. This is called a ring-expansion reaction. Draw a mechanism for the following ring-expansion reaction.

Question: Show two ways to convert an alkyl halide into a carboxylic acid that has one more carbon than the alkyl halide.
What are the products of the following reactions? (A trace amount of acid is present in each case.)
Question: Thiols can be prepared from the reaction of thiourea with an alkyl halide, followed by hydroxide-ion-promoted hydrolysis.
a.Propose a mechanism for the reaction.
b.What thiol will be formed if the alkyl halide employed is pentyl bromide?
Question:Show how each of the following compounds could be prepared from the given starting material. Each requires a protecting group.



What do you think about this solution?
We value your feedback to improve our textbook solutions.