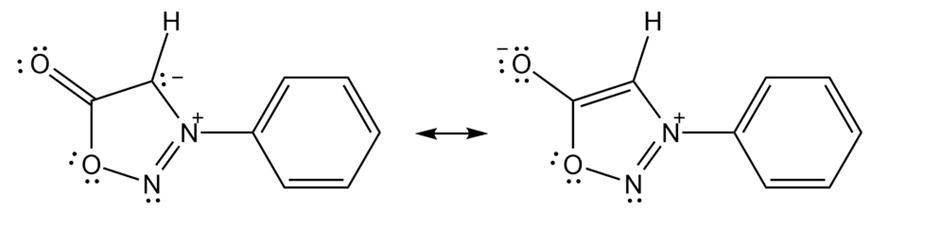
In N-phenylsydnone, there are two rings, one is the benzene ring and the other is the 5-membered. The benzene ring is aromatic in nature.
N-phenylsydnone
Aromaticity according to Huckel rule of the 5-membered ring
The second resonance from of the 5-membered ring of N-phenlsydnone shows the aromaticity. In this form, the ring oxygen from the 5-membered ring contributes two electrons to the ring pi system. There is a contribution of two electrons from one of the nitrogen and there is a contribution of one electron from each carbon. The carbonyl oxygen bears a formal negative charge. So, there is a total of six electrons and hence showing the aromaticity according to the Huckel’s rule. With these six pi electrons, the cyclic conjugated system obeys Huckel’s rule.
Thus, N-phenylsydnone behaves like a typical aromatic molecule obeying Huckel’s electron rule.
 N-phenylsydnone
N-phenylsydnone





