Chapter 19: Q10P (page 486)
Chemical equilibrium and analysis of a mixture. (Warning! This is a long problem.) A remote optical sensor for in the ocean was designed to operate without the need for calibration.33

The sensor compartment is separated from seawater by a silicone membrane through which , but not dissolved ions, can diffuse. Inside the sensor, equilibrates with and . For each
measurement, the sensor is flushed with fresh solution containingbromothymol blue indicator. All indicator is in the formnear neutral pH, so we can
write two mass balances:
has an absorbance maximum at 434 nm andhas a maximum at 620 nm. The sensor measures the absorbance ratio reproducibly without need for calibration. From this ratio, we can findin the seawater as outlined here:
(a).From Beer’s law for the mixture, write equations forin terms of the absorbance at 620 and 434 nmThen show that
(A)
(b) From the mass balance (1) and the acid dissociation constant
, show that
(B)
(C)
(c) Show that (D)
(d) From the carbonic acid dissociation equilibria, show that
(e) Write the charge balance for the solution in the sensor compartment. Substitute in expressions B, C, E, and F forHln,
(f) Suppose that the various constants have the following values:
From the measured absorbance ratio=2.84, findin the seawater.
(g) Approximately what is the ionic strength inside the sensor compartment? Were we justified in neglecting activity coefficients in working this problem?
(e). The charge balance is
(f)
(g). The ionic strength is .
Step by step solution
01
Show that [In2−][HIn−]=RAε434Hnn−ε6,20Hn−2ε620ln2−RAε434ln2=RIn :
Consider,
A as absorbance,
e as molar absorptivity,
b as pathlength.
From the given question we can get,
By solving above equation we get,
Divideby Hln ,

Divide to the numerator and denominator,
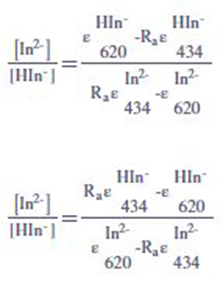
Hence proved that
02
Show that [Hln−]=F1nRln+1 and [ln2−]=KlnFln[H+](Rln+1) :
To prove ,
For indicator, the mass balance can be given as,
Divide Hln both side,
Hence proved
The acid dissociation constant of the indicator is,
Substitute,
03
Show that H+=Kln/Rln :
Hence,
Therefore it has been proved, .
04
Show that [HCO3]=K1[CO(aq) ][H+]and [CO32−]=K1K2CO(aq) ][H+]2:
the acid dissociation reaction of Carbonic acid.
Hence proved
the acid dissociation reaction of Bicarbonate,
Substitute we get,
05
Find charge balance:
06
find CO2(aq) :
To find, The value of is substituted into mass balance
07
Find ionic strength:
The are present in the solution. If the net cation charge is ,the net charge on anion should be and the ionic strength must approximately activity coefficients are close to 1.00 and hence an ionic strength of Mis low .
The solution for ionic strength is 125 .
Unlock Step-by-Step Solutions & Ace Your Exams!
-
Full Textbook Solutions
Get detailed explanations and key concepts
-
Unlimited Al creation
Al flashcards, explanations, exams and more...
-
Ads-free access
To over 500 millions flashcards
-
Money-back guarantee
We refund you if you fail your exam.
Over 30 million students worldwide already upgrade their
learning with Vaia!






