Here is an immunoassay to measure explosives such as trinitrotoluene (TNT) in organic solvent extracts of soil. The assay employs a flow cytometer, which counts small particles (such as living cells) flowing through a narrow tube past a detector. The cytometer in this experiment irradiates the particles with a green
laser and measures fluorescence from each particle as it flows past the detector.
1. Antibodies that bind TNT are chemically attached to 5mmdiameter latex beads.
2. The beads are incubated with a fluorescent derivative of TNT to saturate the antibodies, and excess TNT derivative is removed. The beads are resuspended in aqueous detergent.
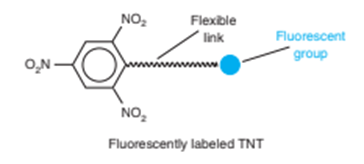
3. 5mlof the suspension are added to 100mlof sample or standard. TNT in the sample or standard displaces some derivatized TNT from bound antibodies. The higher the concentration of TNT, the more derivatized TNT is displaced.
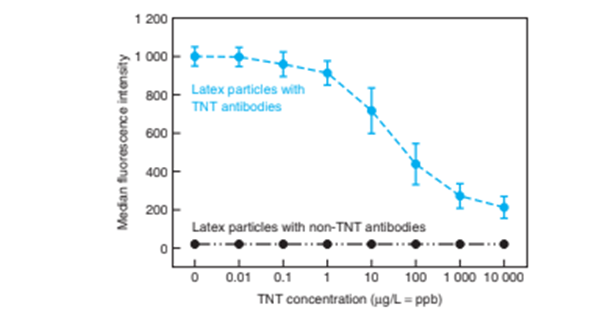
4. An aliquot is injected into the flow cytometer, which measures fluorescence of individual beads as they pass the detector. The figure shows median fluorescence intensity 6 standard deviation. TNT can be quantified in the ppb to ppm range.
Draw pictures showing the state of the beads in steps 1, 2, and 3and explain how this method works.








