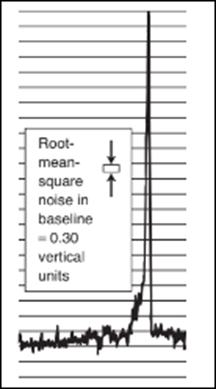
(a) The atomic absorption signal shown here was obtained within a graphite furnace. The root-mean-square noise in the baseline, measured by the instrument's computer, is s=0.30 vertical units, where each horizontal line on the chart is 1 vertical
unit. Estimate where the baseline is beneath the tall signal and measure the height of the signal. Estimate the detection limit for Fe, defined as the concentration of Fe that gives a signal height of 3 s.
![]()
(b) Seven replicate measurements of a standard containinggave readings of 0.88,1.48,0.94,1.12,1.03,1.40, andin cold vapor atomic absorption (Box 21-1). From Equations 5-5 and 5 -6, estimate the detection and quantitation limits. (Note that in Equations 5-5 and 5-6, the quotient s / m is the standard deviation in concentration.)



