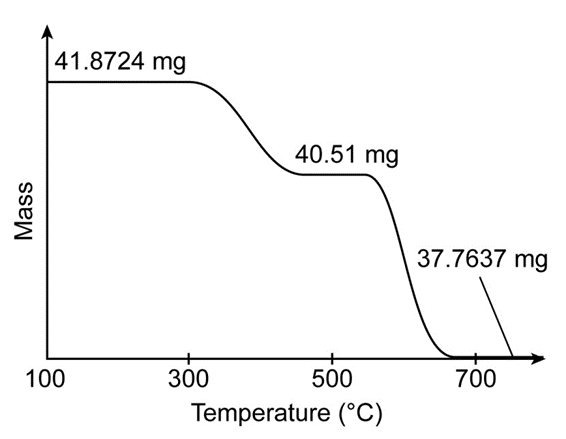
, has a perovskite structure (Box 19-1) with variable oxygen content. The figure shows the thermogravimetric curve observed when 41.8724m gwere heated in role="math" localid="1665035505591" does not react, but cobalt is reduced to Co(s)
(a) What is the oxidation state of cobalt in the ideal formula
(b) Write the reaction of role="math" localid="1665035588740" to produce
(c) If react completely, what will be the mass of product?
(d) Write the balanced reaction of role="math" localid="1665035782894" to produce react completely; what will be the mass of solid product? Your answer will be an expression with x in it.
(e) From the observed product mass , find x in Write the formula of the starting solid.
(f) In part (c), the mass lost from the ideal formula , is 4.0877 mg. What is the product nearrole="math" localid="1665035980286" where the mass is -40.51 mg?