In the given reaction, the needed volume of alcoholic DMG to raise it by 50% excess.
The reaction is,
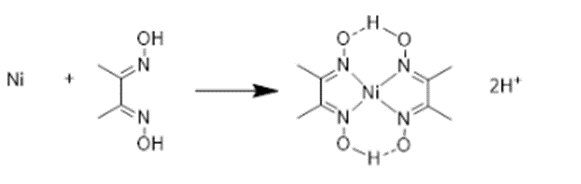
The mass of 2.00Wt% nickel in 1.8g of steel is calculated as
DMG and nickel have a 2 :1 ratio. DMG's mass is estimated as follows:
The excess of 50% of DMG is computed as
The quantity of DMG in 2.15% is calculated as follows:
Hence, the required volume of alcoholic DMG to raise by 50% more is 33mL
The quantity of Ni-DMGcomplex red precipitate is calculated as
Therefore, the Ni-DMG complex red precipitate mass is 0.18 g


