A bonded stationary phase for the separation of optical isomers has the structure
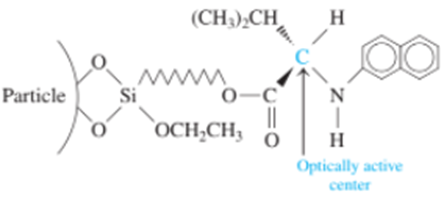
To resolve the enantiomers of amines, alcohols, or thiols, the compounds are first derivatized with a nitroaromatic group that increases their interaction with the bonded phase and makes them observable with a spectrophotometric detector.
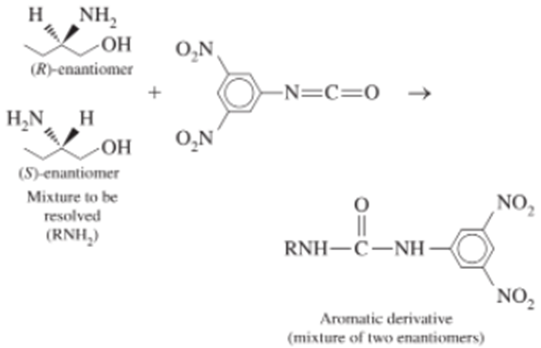
When the mixture is eluted with 20 vol% 2-propanol in hexane, the (R)-enantiomer is eluted before the (S)-enantiomer, with the following chromatographic parameters:
Resolution
Relative retention
k for (R)-isomer =1.35
whereis the average width of the two Gaussian peaks at their base.
(a) Findandwith units of minutes.
(b) The width of a peak at half-height isrole="math" localid="1663582749865" (Figure 23-9). If the plate number for cach peak is the same, findfor each peak.
(c) The area of a Gaussian peak is. Given that the areas under the two bands should be equal, find the relative peak heights.




